Potential utilization of Streptomyces extract as an antibacterial, anti-inflammatory and immune stimulant in Aeromonas hydrophila-infected Nile tilapia, Oreochromis niloticus
Keywords:
Streptomyces, novel antimicrobials, inflammation, immune stimulants, Nile tilapiaAbstract
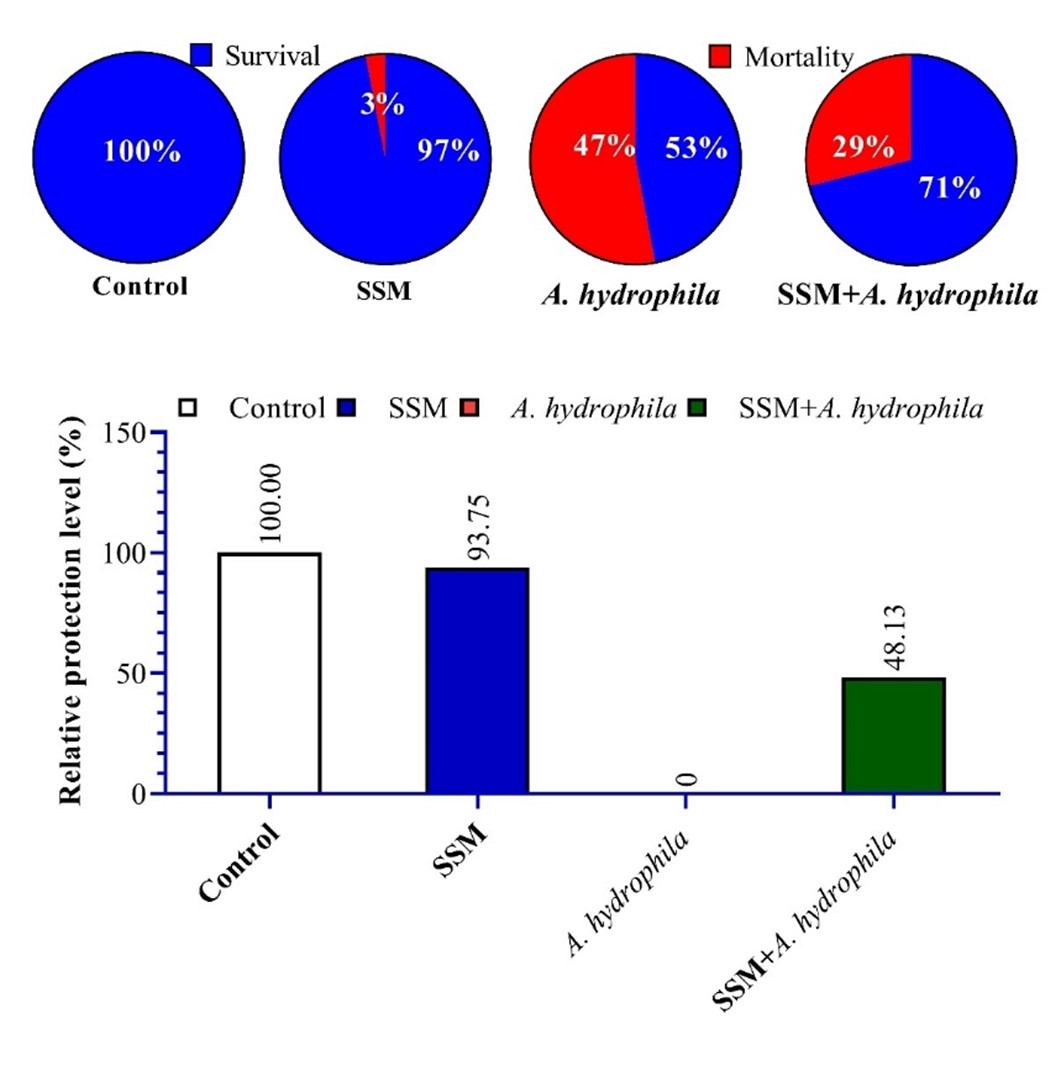
Frequent disease outbreaks in the aquaculture sector have increased with the widespread multiresistant bacterial strains as a result of overuse or misuse of antibiotics. Such diseases can lead to substantial economic losses and threaten the sustainability of aquaculture. Streptomycetes sp. is a genus of bacteria that produces numerous secondary metabolites with high potential as antimicrobial substances. Therefore, the present study aimed to evaluate the use of Streptomyces coeruleorubidus extract (SSM) on survival, immune, and inflammatory responses of Nile tilapia infected with Aeromonas hydrophlia. SSM was extracted from the bacterial media using ethyl acetate, then concentrated using a rotary evaporator, and injected into fish infected with or without A. hydrophlia. The results indicate that infected fish have a lower survival rate and experience low plasma levels of immune components such as IgM, lysozyme, and complement 3 levels. SSM treatment improved the survival rate of infected fish and stimulated immune responses in healthy or infected groups. The relative expression of pro-inflammatory cytokines was significantly increased by infection; meanwhile, anti-inflammatory cytokines were down-regulated. Treatment with SSM significantly regulated the expression of inflammatory cytokines. SSM revealed the potential to stimulate the immune response and improve the survival rate against pathogenic bacterial infection. It can be used and commercialized as an antibacterial agent and an alternative to antibiotics in aquaculture. Future studies could apply these metabolites in human applications; however, more studies are needed to study these metabolites and identify their composition.
References
Abarike, E. D., Jian, J., Tang, J., Cai, J., Sakyi, E. M., & Kuebutornye, F. K. A. (2020). A mixture of Chinese herbs and a commercial probiotic Bacillus species improves hemato-immunological, stress, and antioxidant parameters, and expression of HSP70 and HIF-1α mRNA to hypoxia, cold, and heat stress in Nile tilapia, Oreochromis niloticus. Aquaculture Reports, 18, 100438. https://doi.org/10.1016/j.aqrep.2020.100438.
Abdel‐Latif, H. M., & Khafaga, A. F. (2020). Natural co‐infection of cultured Nile tilapia Oreochromis niloticus with Aeromonas hydrophila and Gyrodactylus cichlidarum experiencing high mortality during summer. Aquaculture Research, 51(5), 1880-1892. https://doi.org/10.1111/are.14538.
Abdelaziz, R., Elsheshtawy, H. M., El-Houseiny, W., Aloufi, A. S., Alwutayd, K. M., Mansour, A. T., Hadad, G., Arisha, A. H., & Yassin, A. M. (2024). A novel metabolite of Streptomyces coeruleorubidus exhibits antibacterial activity against Streptococcus agalactiae through modulation of physiological performance, inflammatory cytokines, apoptosis, and oxidative stress-correlated gene expressions in Nile tilapia (Oreochromis niloticus). Fish & Shellfish Immunology, 148, 109496. https://doi.org/10.1016/j.fsi.2024.109496.
Amábile-Cuevas, C. F. (2016). Antibiotics and antibiotic resistance in the environment. Tylor & Frances group, CRC Press/Balkema book.
Amarasinghe, G. K., Aréchiga Ceballos, N. G., Banyard, A. C., Basler, C. F., Bavari, S., Bennett, A. J., Blasdell, K. R., Briese, T., Bukreyev, A., & Caì, Y. (2018). Taxonomy of the order Mononegavirales: update 2018. Archives of virology, 163, 2283-2294. https://doi.org/10.1007/s00705-018-3814-x.
Ayoub, H. F., Khafagy, A. R., Esawy, A. M., El-Moaty, N. A., Alwutayd, K. M., Mansour, A. T., Ibrahim, R. A., Abdel-Moneam, D. A., & El-Tarabili, R. M. (2024). Phenotypic, molecular detection, and Antibiotic Resistance Profile (MDR and XDR) of Aeromonas hydrophila isolated from Farmed Tilapia zillii and Mugil cephalus. BMC Veterinary Research, 20(1), 84. https://doi.org/10.1186/s12917-024-03942-y.
Berdy, J. (2005). Bioactive microbial metabolites. The Journal of antibiotics, 58(1), 1-26. Bioactive microbial metabolites.
Bérdy, J. J. T. J. o. a. (2012). Thoughts and facts about antibiotics: where we are now and where we are heading. The Journal of antibiotics, 65(8), 385-395. https://doi.org/10.1038/ja.2012.27.
Chen, S.-W., Liu, C.-H., & Hu, S.-Y. (2019). Dietary administration of probiotic Paenibacillus ehimensis NPUST1 with bacteriocin-like activity improves growth performance and immunity against Aeromonas hydrophila and Streptococcus iniae in Nile tilapia (Oreochromis niloticus). Fish & Shellfish Immunology, 84, 695-703. https://doi.org/10.1016/j.fsi.2018.10.059.
Chomczynski, P. (1993). A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques, 15(3), 532-534, 536.
Cochrane, S. A., Lohans, C. T., van Belkum, M. J., Bels, M. A., & Vederas, J. C. (2015). Studies on tridecaptin B 1, a lipopeptide with activity against multidrug resistant Gram-negative bacteria. Organic & biomolecular chemistry, 13(21), 6073-6081. https://doi.org/10.1039/C5OB00780A.
Dahdouh, B., Basha, O., Khalil, S., & Tanekhy, M. (2016). Molecular characterization, antimicrobial susceptibility and salt tolerance of Aeromonas hydrophila from fresh, brackish and marine fishes. Alexandria Journal of Veterinary Sciences, 48, 46-53. https://doi.org/10.5455/ajvs.208107.
Del Carratore, F., Hanko, E. K., Breitling, R., & Takano, E. (2022). Biotechnological application of Streptomyces for the production of clinical drugs and other bioactive molecules. Current Opinion in Biotechnology, 77, 102762. https://doi.org/10.1016/j.copbio.2022.102762.
Dias, M. K., Sampaio, L. S., Proietti-Junior, A. A., Yoshioka, E. T., Rodrigues, D. P., Rodriguez, A. F., Ribeiro, R. A., Faria, F. S., Ozório, R. O., & Tavares-Dias, M. (2016). Lethal dose and clinical signs of Aeromonas hydrophila in Arapaima gigas (Arapaimidae), the giant fish from Amazon. Veterinary microbiology, 188, 12-15. https://doi.org/10.1016/j.vetmic.2016.04.001.
El-Sayed, A.-F. M. (2019). Tilapia culture. 2nd. Academic Press, Elsevier.
El‐Sayed, A. F. M., & Fitzsimmons, K. (2023). From Africa to the world—The journey of Nile tilapia. Reviews in Aquaculture, 15, 6-21. https://doi.org/10.1111/raq.12738.
Elsheshtawy, A., Yehia, N., Elkemary, M., & Soliman, H. (2019). Investigation of Nile tilapia summer mortality in Kafr El-Sheikh governorate, Egypt. Genetics of Aquatic Organisms, 3(1), 17-25. http://doi.org/10.4194/2459-1831-v3_1_03.
FAO. (2021). Global aquaculture production 1950–2019. Food and Agriculture Organization, Roma, Italy. http://www.fao.org/fishery/statistics/global-aquaculture-production/query/en.
Gupta, R., & Sharma, S. (2022). Role of alternatives to antibiotics in mitigating the antimicrobial resistance crisis. Indian Journal of Medical Research, 156(3), 464-477. https://doi.org/10.4103/ijmr.IJMR_3514_20.
Holloway, A., Rao, S., & Shannon, M. (2002). Regulation of cytokine gene transcription in the immune system. Molecular immunology, 38(8), 567-580. https://doi.org/10.1016/S0161-5890(01)00094-3.
Hossain, S., & Heo, G. J. (2021). Ornamental fish: a potential source of pathogenic and multidrug‐resistant motile Aeromonas spp. Letters in Applied Microbiology, 72(1), 2-12. https://doi.org/10.1111/lam.13373.
Inglis, V., Roberts, R., & Bromage, N. (1993). Streptococcal infections.
Kandula, S. K., & Terli, R. (2013). Production, purification and characterization of an antimicrobial compound from marine Streptomyces coeruleorubidus BTSS-301. Journal of pharmacy research, 7(5), 397-403. https://doi.org/10.1016/j.jopr.2013.04.047.
Kumar, K. S., Haritha, R., Swathi, A., Sirisha, B., & Ramana, T. (2012). Taxonomy and Antimicrobial Activity of Streptomyces coeruleorubidus sp. isolated from Marine Sediment. Research Journal of Microbiology, 7(3), 171.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. methods, 25(4), 402-408. https://doi.org/10.1006/meth.2001.1262.
Neamat‐Allah, A. N., Mahsoub, Y. H., & Mahmoud, E. A. (2021). The potential benefits of dietary β‐glucan against growth retardation, immunosuppression, oxidative stress and expression of related genes and susceptibility to Aeromonas hydrophila challenge in Oreochromis niloticus induced by herbicide pendimethalin. Aquaculture Research, 52(2), 518-528. https://doi.org/10.1111/are.14910.
Newman, S., & Majnarich, J. (1982). Direct immersion vaccination of juvenile rainbow trout, Salmo gairdneri Richardson, and juvenile Coho salmon, Oncorhynchus kisutch (Walbaum), with a Yersinia ruckeri bacterin. Journal of Fish Diseases, 5(4), 339-341.
Okon, E. M., Okocha, R. C., Adesina, B. T., Ehigie, J. O., Alabi, O. O., Bolanle, A. M., Matekwe, N., Falana, B. M., Tiamiyu, A. M., & Olatoye, I. O. (2022). Antimicrobial resistance in fish and poultry: Public health implications for animal source food production in Nigeria, Egypt, and South Africa. Frontiers in Antibiotics, 1, 1043302. https://doi.org/10.3389/frabi.2022.1043302.
Ørmen, Ø., Regue, M. Q., Tomás, J. M., & Granum, P. E. (2003). Studies of aerolysin promoters from different Aeromonas spp. Microbial pathogenesis, 35(5), 189-196. https://doi.org/10.1016/S0882-4010(03)00124-4.
Reyes-Cerpa, S., Maisey, K., Reyes-López, F., Toro-Ascuy, D., Sandino, A. M., & Imarai, M. (2012). Fish cytokines and immune response. In New advances and contributions to fish biology (Vol. 1). Books on Demand.
Santos, L., & Ramos, F. (2018). Antimicrobial resistance in aquaculture: Current knowledge and alternatives to tackle the problem. International journal of antimicrobial agents, 52(2), 135-143. https://doi.org/10.1016/j.ijantimicag.2018.03.010.
Sherif, A. H., & Kassab, A. S. (2023). Multidrug-resistant Aeromonas bacteria prevalence in Nile tilapia broodstock. BMC microbiology, 23(1), 80. https://doi.org/10.1186/s12866-023-02827-8.
Sivalingam, P., Hong, K., Pote, J., & Prabakar, K. (2019). Extreme environment Streptomyces: potential sources for new antibacterial and anticancer drug leads? International Journal of Microbiology, 2019, 5283948 https://doi.org/10.1155/2019/5283948.
Standen, B. T., Peggs, D. L., Rawling, M. D., Foey, A., Davies, S. J., Santos, G. A., & Merrifield, D. L. (2016). Dietary administration of a commercial mixed-species probiotic improves growth performance and modulates the intestinal immunity of tilapia, Oreochromis niloticus. Fish & Shellfish Immunology, 49, 427-435. https://doi.org/10.1016/j.fsi.2015.11.037.
Subramani, R., & Sipkema, D. (2019). Marine rare actinomycetes: a promising source of structurally diverse and unique novel natural products. Marine drugs, 17(5), 249. https://doi.org/10.3390/md17050249.
Swain, P., Dash, S., Sahoo, P., Routray, P., Sahoo, S., Gupta, S., Meher, P., & Sarangi, N. (2007). Non-specific immune parameters of brood Indian major carp Labeo rohita and their seasonal variations. Fish & Shellfish Immunology, 22(1-2), 38-43. https://doi.org/10.1016/j.fsi.2006.03.010.
Talebi, B. A. A., Rizvanov, A. A., Haertlé, T., & Blatt, N. L. (2019). World Health Organization report: current crisis of antibiotic resistance. BioNanoScience, 9(4), 778-788. https://doi.org/10.1007/s12668-019-00658-4.
Tan, L. T.-H., Chan, K.-G., Chan, C. K., Khan, T. M., Lee, L.-H., & Goh, B.-H. (2018). Antioxidative potential of a Streptomyces sp. MUM292 isolated from mangrove soil. BioMed research international, 2018, 4823126 https://doi.org/10.1155/2018/4823126.
Tyc, O., Song, C., Dickschat, J. S., Vos, M., & Garbeva, P. (2017). The ecological role of volatile and soluble secondary metabolites produced by soil bacteria. Trends in microbiology, 25(4), 280-292. https://doi.org/10.1016/j.tim.2016.12.002.
Velázquez, J., Acosta, J., Herrera, N., Morales, A., González, O., Herrera, F., Estrada, M. P., & Carpio, Y. (2017). Novel IFNγ homologue identified in Nile tilapia (Oreochromis niloticus) links with immune response in gills under different stimuli. Fish & Shellfish Immunology, 71, 275-285. https://doi.org/10.1016/j.fsi.2017.10.014.
Wangkaghart, E., Deville, S., Wang, B., Srisapoome, P., Wang, T., & Secombes, C. J. (2021). Immune response and protective efficacy of two new adjuvants, Montanide™ ISA 763B VG and Montanide™ GEL02, administered with a Streptococcus agalactiae ghost vaccine in Nile tilapia (Oreochromis niloticus). Fish & Shellfish Immunology, 116, 19-29. https://doi.org/10.1016/j.fsi.2021.06.014.
Wei, Y., Liu, Q., Yu, J., Feng, Q., Zhao, L., Song, H., & Wang, W. (2015). Antibacterial mode of action of 1, 8-dihydroxy-anthraquinone from Porphyra haitanensis against Staphylococcus aureus. Natural product research, 29(10), 976-979. https://doi.org/10.1080/14786419.2014.964705.
Downloads
Published
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
License
Copyright (c) 2025 Loujaina Farhan Abdalmalk (Author)

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright © Author/s 2025 under the terms of the Creative Commons Attribution 4.0 International License.






