The potential role of photobiotic in enhancing liver antioxidant status as determined by enzyme scavenging activity and molecular mechanisms in Gilthead seabream (Sparus aurata) teleost
Keywords:
Moringa leave, liver homogenate, oxidative stress, seabream, antioxidant enzyme, nrf2 pathwaysAbstract
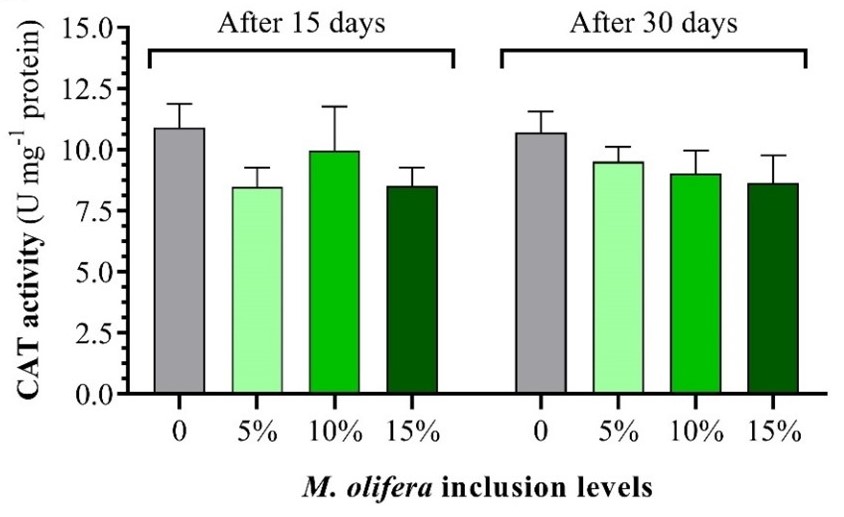
Oxidative stress is one of the intensive aquaculture implications, especially in fish fed high dietary lipids. The use of natural plants as a source of antioxidant substances in the aquatic diet is an alternative natural-based solution to overcome. The present study aims to investigate the potential effects of Moringa olifera leave meal (MLM) on antioxidant status in the liver of Gilthead seabream (Sparus aurata) as determined by antioxidant enzyme activities (CAT, SOD and GR), antioxidant gene expression (CAT, CuZn-SOD, and GR), and regulatory pathway genes (nrf2, nkef-A, and nkef-B). S. aurata specimens (138.75 g) were divided into 4 groups in duplicate and fed MLM at increasing levels of 5, 10, and 15% at a daily feeding rate of 1.5%. Liver samples were collected after 15 and 30 days of intervention. The obtained findings revealed that dietary MLM significantly enhanced the liver activities of SOD and GR enzymes compared to the control group after 15 days of treatment, and the effect continued after 30 days. However, the catalase enzyme remains unaffected throughout the experiment. The gene expression of CuZn-SOD was significantly upregulated after 15 and 30 days of MOL intervention. However, the significant increase of GR gene expression was reported after 15 days only. CAT gene expression tended to increase with dietary MOL. The regulatory gene expression, including nrf2, nkef-A, and nkef-B were significantly upregulated with dietary MOL at levels of 5-10%. In conclusion, the dietary intervention of MOL could enhance liver antioxidant status at both biochemical and molecular levels, and the possible mechanism is enhancing nrf2 pathways as antioxidant regulatory genes.
References
Abdel-Latif, H. M., Abdel-Daim, M. M., Shukry, M., Nowosad, J., & Kucharczyk, D. (2022). Benefits and applications of Moringa oleifera as a plant protein source in Aquafeed: A review. Aquaculture, 547, 737369.
Abdelaziz, R., Elsheshtawy, H. M., El-Houseiny, W., Aloufi, A. S., Alwutayd, K. M., Mansour, A. T., . . . Yassin, A. M. (2024). A novel metabolite of Streptomyces coeruleorubidus exhibits antibacterial activity against Streptococcus agalactiae through modulation of physiological performance, inflammatory cytokines, apoptosis, and oxidative stress-correlated gene expressions in Nile tilapia (Oreochromis niloticus). Fish & shellfish immunology, 148, 109496.
Ahmadifar, E., Pourmohammadi Fallah, H., Yousefi, M., Dawood, M. A., Hoseinifar, S. H., Adineh, H., . . . Doan, H. V. (2021). The gene regulatory roles of herbal extracts on the growth, immune system, and reproduction of fish. Animals, 11(8), 2167. https://doi.org/https://doi.org/10.3390/ani11082167
Ahmed, S. A., Ibrahim, R. E., Younis, E. M., Abdelwarith, A. A., Faroh, K. Y., El Gamal, S. A., . . . Davies, S. J. (2024). Antagonistic effect of zinc oxide nanoparticles dietary supplementation against chronic copper waterborne exposure on growth, behavioral, biochemical, and gene expression alterations of African catfish, Clarias gariepinus (Burchell, 1822). Biological Trace Element Research, 202(12), 5697-5713.
Almarri, S. H., Khalil, A. A., Mansour, A. T., & El-Houseiny, W. (2023). Antioxidant, Immunostimulant, and Growth-Promoting Effects of Dietary Annona squamosa Leaf Extract on Nile Tilapia, Oreochromis niloticus, and Its Tolerance to Thermal Stress and Aeromonas sobria Infection. Animals, 13(4), 746. https://doi.org/https://doi.org/10.3390/ani13040746
Alwaleed, E. A., Alotaibi, N. M., Mansour, A. T., Alghamdi, M. A., & Abdelgaliel, A. S. (2024). Assessment of the conceivable inhibitory activity of pathogenic microorganisms extracted from seaweed using phytochemicals, antioxidants, and in-silico molecular dynamic simulation. Scientific Reports, 14(1), 23200. https://doi.org/https://doi.org/10.1038/s41598-024-70620-2
Anwar, F., Latif, S., Ashraf, M., & Gilani, A. H. (2007). Moringa oleifera: a food plant with multiple medicinal uses. Phytotherapy research, 21(1), 17-25.
AOAC. (2000). Association of Official Analytical Chemists. International Official methods of Analysis. 17th edition.
Biswas, S. K. (2016). Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxidative medicine and cellular longevity, 2016(1), 5698931. https://doi.org/ https://doi.org/10.1155/2016/5698931
Chomczynski, P. (1993). A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques, 15(3), 532-534, 536-537.
Chumark, P., Khunawat, P., Sanvarinda, Y., Phornchirasilp, S., Morales, N. P., Phivthong-ngam, L., . . . Klai-upsorn, S. P. (2008). The in vitro and ex vivo antioxidant properties, hypolipidaemic and antiatherosclerotic activities of water extract of Moringa oleifera Lam. leaves. Journal of ethnopharmacology, 116(3), 439-446.
Dongmeza, E., Siddhuraju, P., Francis, G., & Becker, K. (2006). Effects of dehydrated methanol extracts of moringa (Moringa oleifera Lam.) leaves and three of its fractions on growth performance and feed nutrient assimilation in Nile tilapia (Oreochromis niloticus (L.)). Aquaculture, 261(1), 407-422.
Dzuvor, C. K., Pan, S., Amanze, C., Amuzu, P., Asakiya, C., & Kubi, F. (2022). Bioactive components from Moringa oleifera seeds: production, functionalities and applications–a critical review. Critical Reviews in Biotechnology, 42(2), 271-293. https://doi.org/https://doi.org/10.1080/07388551.2021.1931804
El-Houseiny, W., Abdelaziz, R., Mansour, A. T., Alqhtani, H. A., Bin-Jumah, M. N., Bayoumi, Y., . . . Al-Sagheer, A. A. (2025). Effects of α-sitosterol on growth, hematobiochemical profiles, immune-antioxidant resilience, histopathological features and expression of immune apoptotic genes of Nile tilapia, Oreochromis niloticus, challenged with Candida albicans. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 275, 111035. https://doi.org/https://doi.org/10.1016/j.cbpb.2024.111035
El-Houseiny, W., Anter, R. G., Arisha, A. H., Mansour, A. T., Safhi, F. A., Alwutayd, K. M., . . . Mohamed, E. M. (2023). Growth Retardation, Oxidative Stress, Immunosuppression, and Inflammatory Disturbances Induced by Herbicide Exposure of Catfish, Clarias gariepinus, and the Alleviation Effect of Dietary Wormwood, Artemisia cina. Fishes, 8(6), 297. https://doi.org/https://doi.org/10.3390/fishes8060297
El-Kassas, S., Aljahdali, N., Abdo, S. E., Alaryani, F. S., Moustafa, E. M., Mohamed, R., . . . Shafi, M. E. (2022). Moringa oleifera leaf powder dietary inclusion differentially modulates the antioxidant, inflammatory, and histopathological responses of normal and Aeromonas hydrophila-infected mono-sex nile tilapia (Oreochromis niloticus). Frontiers in veterinary science, 9, 918933. https://doi.org/https://doi.org/10.3389/fvets.2022.918933
Faheem, M., Khaliq, S., Abbas, R. Z., & Mansour, A. T. (2022). Moringa oleifera alleviated oxidative stress, physiological and molecular disruption induced by acute thermal stress in grass carp, Ctenopharyngodon idella. Fish Physiology and Biochemistry, 48, 1463–1473. https://doi.org/https://doi.org/10.1007/s10695-022-01147-4
FAO. (2016). Food and Agriculture Organization. Fishery and Aquaculture Statistics, Aquaculture Production. Yearbook.
Frewer, L., Kole, A., Van De Kroon, S., & De Lauwere, C. (2005). Consumer attitudes towards the development of animal-friendly husbandry systems. Journal of Agricultural and Environmental Ethics, 18(4), 345-367.
Fuglie, L. J. (1999). The Miracle Tree: Moringa oleifera: natural nutrition for the Tropics, Church World Service, Dakar, p.68; Revised in 2001 and published as
The Miracle Tree: The Multiple Attributes of Moringa. p. 172.
García-Beltrán, J. M., Mansour, A. T., Alsaqufi, A. S., Ali, H. M., & Esteban, M. Á. (2020). Effects of aqueous and ethanolic leaf extracts from drumstick tree (Moringa oleifera) on gilthead seabream (Sparus aurata L.) leucocytes, and their cytotoxic, antitumor, bactericidal and antioxidant activities. Fish & shellfish immunology, 106, 44-55. https://doi.org/https://doi.org/10.1016/j.fsi.2020.06.054
González‐Silvera, D., Cuesta, A., & Esteban, M. Á. (2021). Immune defence mechanisms presented in liver homogenates and bile of gilthead seabream (Sparus aurata). Journal of Fish Biology, 99(6), 1958-1967. https://doi.org/https://doi.org/10.1111/jfb.14901
Guevara, A. P., Vargas, C., Sakurai, H., Fujiwara, Y., Hashimoto, K., Maoka, T., . . . Nishino, H. (1999). An antitumor promoter from Moringa oleifera Lam. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 440(2), 181-188.
Hamed, H. S., Amen, R. M., Elelemi, A. H., Mahboub, H. H., Elabd, H., Abdelfattah, A. M., . . . Yassin, E. M. M. (2022). Effect of dietary Moringa oleifera leaves nanoparticles on growth performance, physiological, immunological responses, and liver antioxidant biomarkers in nile tilapia (Oreochromis niloticus) against Zinc oxide nanoparticles toxicity. Fishes, 7(6), 360.
He, J., Wu, X., Huang, S., Wang, J., Niu, S., Chen, M., . . . Hong, B. (2022). Phenolic metabolites from a deep-sea-derived fungus Aspergillus puniceus A2 and their Nrf2-dependent anti-inflammatory effects. Marine Drugs, 20(9), 575. https://doi.org/https://doi.org/10.3390/md20090575
Hussain, T., Tan, B., Yin, Y., Blachier, F., Tossou, M. C., & Rahu, N. (2016). Oxidative stress and inflammation: what polyphenols can do for us? Oxidative medicine and cellular longevity, 2016(1), 7432797. https://doi.org/ https://doi.org/10.1155/2016/7432797
Ibrahim, R. E., Ghamry, H. I., Althobaiti, S. A., Almalki, D. A., Shakweer, M. S., Hassan, M. A., . . . Ahmed, S. A. (2022). Moringa oleifera and Azadirachta indica Leaves enriched diets mitigate chronic oxyfluorfen toxicity induced immunosuppression through disruption of pro/anti-inflammatory gene pathways, alteration of antioxidant gene expression, and histopathological Alteration in Oreochromis niloticus. Fishes, 8(1), 15. https://doi.org/https://doi.org/10.3390/fishes8010015
Ibrahim, R. E., Rhouma, N. R., Elbealy, M. A., Abdelwarith, A. A., Younis, E. M., Khalil, S. S., . . . El-Murr, A. (2024). Effect of dietary intervention with Capsicum annuum extract on growth performance, physiological status, innate immune response, and related gene expression in Nile tilapia. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 270, 110914.
Iqbal, S., & Bhanger, M. (2006). Effect of season and production location on antioxidant activity of Moringa oleifera leaves grown in Pakistan. Journal of food Composition and Analysis, 19(6), 544-551.
Jian, J., & Wu, Z. (2003). Effects of traditional Chinese medicine on nonspecific immunity and disease resistance of large yellow croaker, Pseudosciaena crocea (Richardson). Aquaculture, 218(1), 1-9.
Jovanović, M., Tenji, D., Nikolić, B., Srdić-Rajić, T., Svirčev, E., & Mitić-Ćulafić, D. (2021). In vitro study of two edible Polygonoideae plants: phenolic profile, cytotoxicity, and modulation of Keap1-Nrf2 gene expression. Foods, 10(4), 811. https://doi.org/https://doi.org/10.3390/foods10040811
Kashyap, P., Kumar, S., Riar, C. S., Jindal, N., Baniwal, P., Guiné, R. P., . . . Kumar, H. (2022). Recent advances in Drumstick (Moringa oleifera) leaves bioactive compounds: Composition, health benefits, bioaccessibility, and dietary applications. Antioxidants, 11(2), 402. https://doi.org/https://doi.org/10.3390/antiox11020402
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods, 25(4), 402-408.
Luo, J.-H., Li, J., Shen, Z.-C., Lin, X.-F., Chen, A.-Q., Wang, Y.-F., . . . Wang, X.-Y. (2023). Advances in health-promoting effects of natural polysaccharides: Regulation on Nrf2 antioxidant pathway. Frontiers in Nutrition, 10, 1102146. https://doi.org/ https://doi.org/10.3389/fnut.2023.1102146
Mansour, A. T., Arisha, A. H., Abdelaziz, R., Alwutayd, K. M., Van Doan, H., El-Murr, A. E., & El-Houseiny, W. (2024). Effects of extended dietary supplementation with Santalum album essential oil on hemato-biochemical changes, innate immune response, antioxidant status, and expression of related gene in Nile tilapia (Oreochromis niloticus). Fish Physiology and Biochemistry, 50(3), 955-971. https://doi.org/https://doi.org/10.1007/s10695-024-01309-6
Mansour, A. T., Espinosa, C., García-Beltrán, J. M., Miao, L., Francisco, D. C. C., Alsaqufi, A. S., & Esteban, M. Á. (2020). Dietary supplementation of drumstick tree, Moringa oleifera, improves mucosal immune response in skin and gills of seabream, Sparus aurata, and attenuates the effect of hydrogen peroxide exposure. Fish. Physiol. Bioch., 46(3), 981-996. https://doi.org/https://doi.org/10.1007/s10695-020-00763-2
Mansour, A. T., Mahboub, H. H., Elshopakey, G. E., Aziz, E. K., Alhajji, A. H., Rayan, G., . . . El-Houseiny, W. (2022). Physiological Performance, Antioxidant and Immune Status, Columnaris Resistance, and Growth of Nile Tilapia That Received Alchemilla vulgaris-Supplemented Diets. Antioxidants, 11(8), 1494. https://doi.org/https://doi.org/10.3390/antiox11081494
Mesa-Garcia, M. D., Plaza-Diaz, J., & Gomez-Llorente, C. (2018). Molecular basis of oxidative stress and inflammation. In Obesity (pp. 41-62). Elsevier. https://doi.org/https://doi.org/10.1016/B978-0-12-812504-5.00003-9
Momın, M., & Memiş, D. (2023). Potential use of the miracle tree (Moringa oleifera) leaves in aquaculture: A recent update. Aquatic Sciences and Engineering, 38(2), 122-130. https://doi.org/https://doi.org/10.26650/ASE20221225220
Murakami, A., Kitazono, Y., Jiwajinda, S., Koshimizu, K., & Ohigashi, H. (1998). Niaziminin, a thiocarbamate from the leaves of Moringa oleifera, holds a strict structural requirement for inhibition of tumor-promoter-induced Epstein-Barr virus activation. Planta Medica, 64(04), 319-323.
NRC. (1993). National Research Council, Nutrient Requirements of Fishes National Academy of Sciences.
Oh, H. Y., Lee, T. H., Lee, D.-Y., Lee, C.-H., Joo, M.-S., Kim, H. S., & Kim, K.-D. (2022). Dietary Supplementation with Ginger (Zingiber officinale) Residue from juice extraction improves juvenile black rockfish (Sebastes schlegelii) growth performance, antioxidant enzyme activity, and resistance to Streptococcus iniae infection. Animals, 12(5), 546.
Sola, L., Moretti, A., Crosetti, D., Karaiskou, N., Magoulas, A., Rossi, A., . . . Tsigenopoulos, C. (2006). Gilthead seabream—Sparus aurata. Proceedings of the WP1 workshop on Genetics of domestication, breeding and enhancement of performance of fish and shellfish, Viterbo, Italy,
Tabassum, S., Hussain, S., Ali, S., Arsalan, M. Z.-u.-H., Ahmad, B., Asrar, M., & Sharif, A. (2021). Partial replacement of fish meal with Moringa oleifera leaf meal in practical diets of Cirrhinus mrigala fingerlings. Brazilian Journal of Biology, 83, e246333.
Thirunavukkarasar, R., Kumar, P., Sardar, P., Sahu, N. P., Harikrishna, V., Singha, K. P., . . . Krishna, G. (2022). Protein-sparing effect of dietary lipid: Changes in growth, nutrient utilization, digestion and IGF-I and IGFBP-I expression of Genetically Improved Farmed Tilapia (GIFT), reared in Inland Ground Saline Water. Animal Feed Science and Technology, 284, 115150. https://doi.org/https://doi.org/10.1016/j.anifeedsci.2021.115150
Tocher, D. R., Mourente, G., Van der Eecken, A., Evjemo, J. O., Diaz, E., Wille, M., . . . Olsen, Y. (2003). Comparative study of antioxidant defence mechanisms in marine fish fed variable levels of oxidised oil and vitamin E. Aquaculture International, 11(1), 195-216.
Xu, J., Xie, S., Chi, S., Zhang, S., Cao, J., & Tan, B. (2022). Short-term dietary antibiotics altered the intestinal microbiota and improved the lipid metabolism in hybrid grouper fed medium and high-lipid diets. Aquaculture, 547, 737453. https://doi.org/https://doi.org/10.1016/j.aquaculture.2021.737453
Xu, T., Hu, S., Liu, Y., Sun, K., Luo, L., & Zeng, L. (2022). Hawk tea flavonoids as natural hepatoprotective agents alleviate acute liver damage by reshaping the intestinal microbiota and modulating the Nrf2 and NF-κB signaling pathways. Nutrients, 14(17), 3662. https://doi.org/https://doi.org/10.3390/nu14173662
Xu, W., Lu, H., Yuan, Y., Deng, Z., Zheng, L., & Li, H. (2022). The antioxidant and anti-inflammatory effects of flavonoids from propolis via Nrf2 and NF-κB pathways. Foods, 11(16), 2439. https://doi.org/https://doi.org/10.3390/foods11162439
Yu, L., Wang, Y., Wen, H., Jiang, M., Wu, F., & Tian, J. (2021). Synthesis and evaluation of acetylferulic paeonol ester and ferulic paeonol ester as potential antioxidants to inhibit fish oil oxidation. Food Chemistry, 365, 130384. https://doi.org/https://doi.org/10.1016/j.foodchem.2021.130384
Zahran, E., Elbahnaswy, S., Ahmed, F., Risha, E., Mansour, A. T., Alqahtani, A. s., . . . Sebaei, M. G. E. (2024). Dietary microalgal-fabricated selenium nanoparticles improve Nile tilapia biochemical indices, immune-related gene expression, and intestinal immunity. BMC Veterinary Research, 20(1), 107. https://doi.org/https://doi.org/10.1186/s12917-024-03966-4
Zahran, E., Elbahnaswy, S., Elsayed, M., Saif, N. A., Elhadidy, M., Risha, E., . . . Ahmed, F. (2025). Fabrication of Algogenic Zinc Nanoparticles and Assessment of Their Biomimetics Attributes and Potential Antibacterial Efficacy Against Fish Pathogens. Aquaculture Research, 2025(1), 6304377. https://doi.org/ https://doi.org/10.1155/are/6304377
Downloads
Published
Data Availability Statement
The authors declare that data can be provided by corresponding author upon reasonable request.
License
Copyright (c) 2025 Abdallah Tageldein Mansour, Cristóbal Espinosa, Sabrin Morshidy, M. Ángeles Esteban (Author)

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright © Author/s 2025 under the terms of the Creative Commons Attribution 4.0 International License.






